Cool Info About What Is Potential Difference Between Batteries
Unlocking the Power Within
Ever wondered what makes your phone light up, your car start, or your flashlight shine brightly in the dark? It all boils down to something called "potential difference" in batteries. Don't let the fancy name scare you. Think of it as the electrical "push" or "oomph" that makes electrons flow and power your devices. It's the reason your battery isn't just a useless lump of metal and chemicals.
To really grasp it, let's imagine a water slide. At the top, the water has high potential energy — it's ready to go! At the bottom, the potential energy is lower. The difference in height creates the "push" that sends you zooming down. Potential difference in a battery is similar, but instead of water, we're talking about electrons, and instead of height, we're talking about electrical potential. It's the difference in electrical "pressure" between the positive and negative terminals that drives those tiny electrons through your circuits.
So, the next time you pop a battery into your remote, remember the water slide. The potential difference is the invisible force that's doing all the work, turning chemical energy into the electricity that powers your entertainment (or whatever you're watching on TV!). It's like the unsung hero of the electronic world, quietly working behind the scenes.
Without that potential difference, a battery is just a paperweight. The bigger the potential difference, generally speaking, the more "oomph" the battery can deliver. This is why a 12-volt car battery can start an engine while a 1.5-volt AA battery powers your TV remote. It's all about that electrical "push"! This difference is measured in volts (V), hence the term "voltage."
1. Understanding Volts
Alright, so we've established that potential difference is the "electrical push." But how do we measure this push? That's where volts come in! A volt (V) is the unit we use to quantify potential difference. Think of it like measuring water pressure in a pipe — volts tell us how strongly the electrons are being pushed through the circuit.
A higher voltage means a stronger push, which translates to more electrical power. That's why a wall socket has a much higher voltage (usually 120V or 240V) than a small battery (like 1.5V). The higher voltage is needed to power more demanding appliances like refrigerators and ovens.
It's important to choose the right voltage for your devices. Using a battery with too low a voltage might not provide enough power to operate the device properly. Conversely, using a battery with too high a voltage could damage the device by overloading its circuits. Think of it like trying to use a firehose to water a delicate flower not a good idea!
Voltages are usually clearly marked on batteries and devices. Always double-check to make sure you're using the correct voltage to avoid any electrical mishaps or fried gadgets! Ignoring this crucial piece of information can result in some serious tech-related sadness.
The Inner Workings
So, where does this potential difference actually come from inside a battery? It's not magic, although it might seem that way! It's actually a carefully orchestrated chemical reaction happening within the battery's cells.
Batteries are designed with two different materials that are chemically eager to react with each other. These materials are called electrodes (the anode and the cathode), and they're submerged in a special sauce called an electrolyte. This electrolyte acts like a mediator, helping the chemical reaction along.
When the battery is connected to a circuit, the chemical reaction starts. One electrode (the anode) releases electrons, while the other electrode (the cathode) accepts them. This creates a difference in electrical charge between the two electrodes — voila, potential difference! The electrons then flow through the circuit, powering your device, before returning to the battery to complete the loop. It's a continuous cycle until the chemical reactants are used up, and the battery is "dead."
The specific chemical reaction depends on the type of battery. For example, in a typical alkaline battery, zinc and manganese dioxide react. In a lithium-ion battery (like those in your phone), lithium ions move between the electrodes. But the fundamental principle is the same: a chemical reaction creating a separation of electrical charge, resulting in potential difference, which then drives the flow of electrons.
2. Different Battery Types, Different Potential Differences
Not all batteries are created equal — they come in different types, each with its own unique chemistry and potential difference. This is why you can't just swap any old battery into any device. Different devices require different voltages and current capacities.
Alkaline batteries (like AA and AAA) are common for everyday devices like remotes and toys, typically providing 1.5V. Lithium-ion batteries, found in smartphones and laptops, offer higher energy density and longer lifespans, with voltages varying depending on the specific battery pack configuration. Lead-acid batteries, used in cars, deliver a hefty 12V to crank your engine.
The type of chemicals used and the design of the battery determine its voltage and capacity (how much energy it can store). Some batteries are rechargeable, meaning the chemical reaction can be reversed, allowing you to reuse them multiple times. Others are non-rechargeable and must be disposed of properly after use.
Understanding the different types of batteries and their potential differences is important for choosing the right battery for your needs and ensuring safe operation of your devices. Always consult the device's manual to determine the correct battery type and voltage.

Difference Between Cell And Battery Electrical Volt
Potential Difference and Battery Life
So, potential difference gets things started, but what happens to it over time? Does it stay constant, or does it change as the battery is used? The answer is...it changes! As a battery discharges, the chemical reaction progresses, gradually reducing the amount of reactants available. This, in turn, reduces the potential difference between the electrodes.
Think of it like squeezing a tube of toothpaste. At first, it's easy to squeeze out a lot of toothpaste with minimal effort. But as the tube empties, you have to squeeze harder and harder to get the same amount out. Similarly, as a battery discharges, the potential difference decreases, making it harder to deliver the same amount of current to your device.
Eventually, the potential difference drops below a certain threshold, and the device stops working, even though there might still be some chemical reactants left in the battery. This is because the "electrical push" is no longer strong enough to drive the electrons through the circuit. That's when you know it's time to replace the battery!
Factors like temperature, usage patterns, and the battery's internal resistance can also affect how quickly the potential difference decreases. Extreme temperatures can accelerate the chemical reaction, shortening battery life. High current demands can also drain the battery faster. And internal resistance acts like friction, reducing the efficiency of the battery and causing it to lose potential difference more quickly.
3. Extending Battery Life
Want to squeeze the most out of your batteries? Here are a few tips to help you extend their lifespan and get the most bang for your buck:
First, store batteries in a cool, dry place. Extreme temperatures can drain them even when they're not being used. Second, avoid leaving devices turned on unnecessarily. Even in standby mode, they're still drawing power and draining the battery. Third, use the correct type of battery for each device. Using an incompatible battery can damage the device or shorten the battery's lifespan. And finally, for rechargeable batteries, avoid completely discharging them before recharging. Partial charges are often better for their long-term health.
By following these simple tips, you can help maintain a healthy potential difference within your batteries and extend their usable life. This not only saves you money but also reduces the environmental impact of disposable batteries.
Basically, be nice to your batteries, and they'll be nice to you (by powering your stuff!).

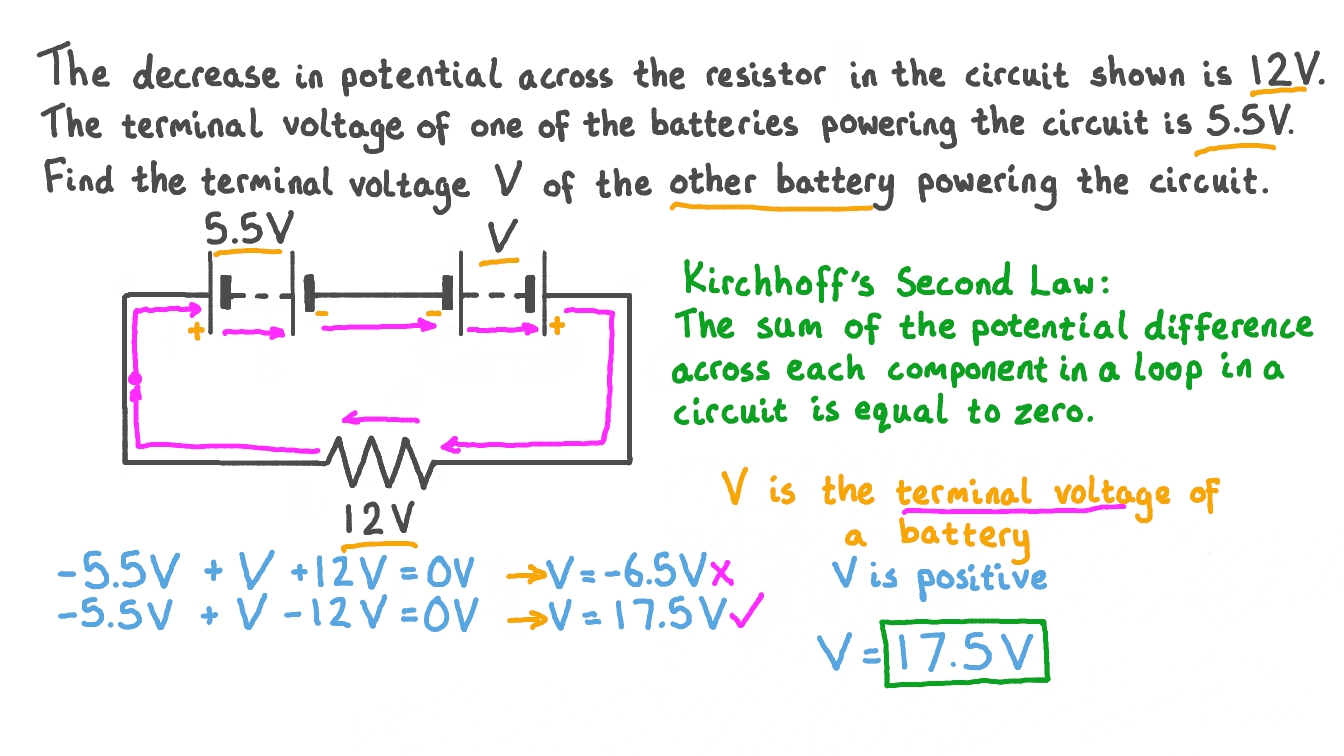
Potential Difference Circuit Diagram Ci
FAQs About Potential Difference and Batteries
4. Q
A: Connecting batteries in series increases the overall potential difference. For example, connecting two 1.5V batteries in series will give you a total of 3V. However, it's crucial to connect them correctly (positive to negative) to avoid short circuits or damage.
5. Q
A: Yes, you can! You'll need a multimeter, which is a handy tool for measuring voltage, current, and resistance. Simply connect the multimeter probes to the positive and negative terminals of the battery, and the meter will display the voltage, which represents the potential difference.
6. Q
A: Battery leakage occurs when the chemical reaction inside the battery produces gases that build up pressure. This pressure can eventually cause the battery casing to rupture, releasing the corrosive electrolyte. It's best to remove batteries from devices that won't be used for extended periods to prevent leakage and potential damage.

Fuel Cells Vs Batteries What’s The Difference? Eco Experts
